

There are nearly ten million known carbon compounds and an entire branch of chemistry, known as organic chemistry, is devoted to their study. For example, if the concentration of carbon-14 in the remains of an organism is half of the natural concentration of carbon-14, a scientist would estimate that the organism died about 5,730 years ago, the half-life of carbon-14. By measuring the percentage of carbon-14 in the remains of an organism, and by assuming that the natural abundance of carbon-14 has remained constant over time, scientists can estimate when that organism died. The carbon-14 within that organism is no longer replaced and the percentage of carbon-14 begins to decrease as it decays.

Once an organism dies, it no longer ingests much of anything. Living things tend to ingest materials that contain carbon, so the percentage of carbon-14 within living things is the same as the percentage of carbon-14 in the environment. Although carbon-14 decays into nitrogen-14 through beta decay, the amount of carbon-14 in the environment remains constant because new carbon-14 is always being created in the upper atmosphere by cosmic rays. Scientists know that a small amount of naturally occurring carbon is carbon-14. The theory behind carbon dating is fairly simple.
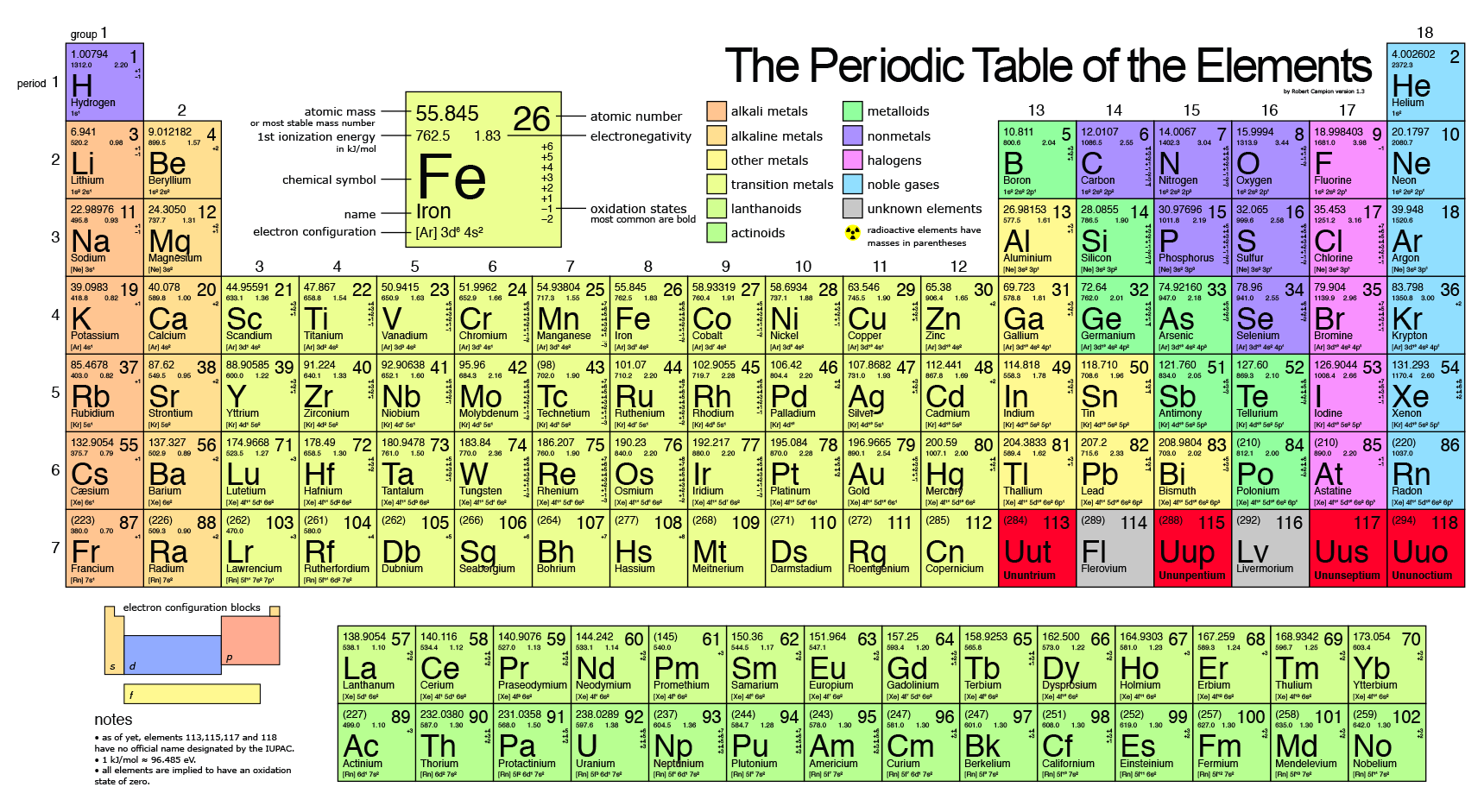
They can trap other atoms within their framework, appear to be capable of withstanding great pressures and have magnetic and superconductive properties.Ĭarbon-14, a radioactive isotope of carbon with a half-life of 5,730 years, is used to find the age of formerly living things through a process known as radiocarbon dating. A single buckyball consists of 60 or 70 carbon atoms (C 60 or C 70) linked together in a structure that looks like a soccer ball. Large molecules consisting only of carbon, known as buckminsterfullerenes, or buckyballs, have recently been discovered and are currently the subject of much scientific interest. Very little is known about this form of carbon. It is a transparent material that can split a single beam of light into two beams, a property known as birefringence. Although they posses very different physical properties, graphite and diamond differ only in their crystal structure.Ī fourth allotrope of carbon, known as white carbon, was produced in 1969. These small diamonds are made by squeezing graphite under high temperatures and pressures for several days or weeks and are primarily used to make things like diamond tipped saw blades. Although naturally occurring diamond is typically used for jewelry, most commercial quality diamonds are artificially produced. Although commonly called lead, the black material used in pencils is actually graphite.ĭiamond, the third naturally occurring form of carbon, is one of the hardest substances known. Coke is made by heating soft coal in an oven without allowing oxygen to mix with it. In addition to its use as a lubricant, graphite, in a form known as coke, is used in large amounts in the production of steel. All artificially produced graphite is of the alpha type. These two forms have identical physical properties but different crystal structures. Naturally occurring graphite occurs in two forms, alpha and beta. Although it does occur naturally, most commercial graphite is produced by treating petroleum coke, a black tar residue remaining after the refinement of crude oil, in an oxygen-free oven. Graphite, one of the softest materials known, is a form of carbon that is primarily used as a lubricant. It can also be pressed into shapes and is used to form the cores of most dry cell batteries, among other things. This black soot, also known as lampblack, gas black, channel black or carbon black, is used to make inks, paints and rubber products.
Three naturally occurring allotropes of carbon are known to exist: amorphous, graphite and diamond.Īmorphous carbon is formed when a material containing carbon is burned without enough oxygen for it to burn completely. Carbon is most commonly obtained from coal deposits, although it usually must be processed into a form suitable for commercial use. Carbon, the sixth most abundant element in the universe, has been known since ancient times.


 0 kommentar(er)
0 kommentar(er)
